












5-Part Auto Hematology Analyzer
IVD device
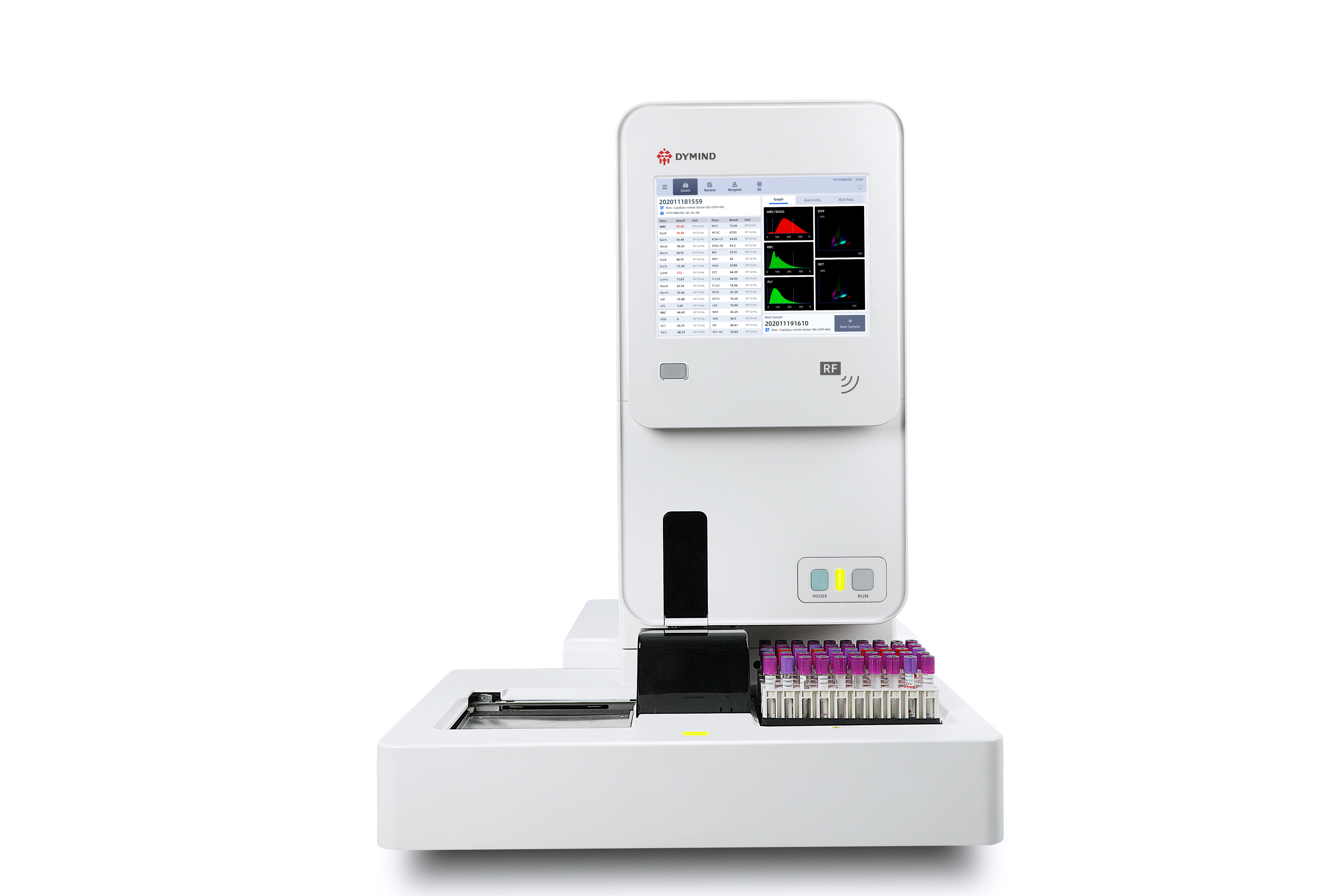
Shenzhen Dymind Biotechnology Co., Ltd.The Dymind DH-615 series adopts fluorescent optics to improve the identification of abnormal cells. The detection of reticulocytes enhances testing for bone marrow erythropoiesis and related diseases. The instrument supports automated sampling of venous and capillary whole blood, which reduces the pressure of blood collection while increasing the detection velocity and reducing the risk of biological contamination. The DH615 series adopts self-developed software to achieve full-process management, which provides accurate and fast test results for the outpatient and emergency laboratory without manual operation.
Client / ManufacturerDesign
Shenzhen Dymind Biotechnology Co., Ltd.
Shenzhen, CNShenzhen Dymind Biotechnology Co., Ltd.
Shenzhen, CNDate of Launch
2020
Development Time
13 - 24 months
Target Regions
Asia
Target Groups
Consumer / User